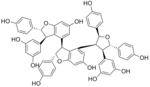
Kobophenol A
The topic of Kobophenol A is one of the most relevant today, since it has a significant impact on our society. There are numerous aspects to consider when addressing this topic, from its historical origin to its current implications. In this article, we will explore different perspectives and points of view on Kobophenol A, with the aim of offering a complete and balanced view. We will analyze the different aspects that make up this topic, as well as its possible consequences in the personal, social and political sphere. Additionally, we will examine how Kobophenol A has evolved over time and how it continues to influence our lives today.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
(2S,2′R,3S,3′R)-3′-(3,5-Dihydroxyphenyl)-4--2,2′-bis(4-hydroxyphenyl)-2,2′,3,3′-tetrahydro-6,6′-diol | |||
| Other names
kob A
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C56H44O13 | |||
| Molar mass | 924.94 g/mol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Kobophenol A is a stilbenoid. It is a tetramer of resveratrol. It can be isolated from Caragana chamlagu, from Caragana sinica and from Carex folliculata seeds.
The molecule shows a 2,3,4,5-tetraaryltetrahydrofuran skeleton.
It has been shown to inhibit acetylcholinesterase.
Acid-catalyzed epimerization of kobophenol A to carasinol B can be performed in vitro.
References
- ^ a b (+)-α-Viniferin, a Stilbene Trimer from Caragana chamlague, Inhibits Acetylcholinesterase. Sang Hyun Sung, So Young Kang, Ki Yong Lee, Mi Jung Park, Jeong Hun Kim, Jong Hee Park, Young Chul Kim, Jinwoong Kim and Young Choong Kim, Biological & Pharmaceutical Bulletin, Vol. 25, 2002 [permanent dead link]
- ^ Simultaneous determination of the contents of three stilbene oligomers in Caragana sinica collected in different seasons using an improved HPLC method. Shu Na; Zhou Hong; Hu Changqi, Biological & pharmaceutical bulletin, 2006, vol. 29, no4, pp. 608-612
- ^ a b Identification and bioactivities of resveratrol oligomers and flavonoids from Carex folliculata seeds. Li L, Henry GE and Seeram NP, J Agric Food Chem., 26 August 2009, volume 57, issue 16, pages 7282-7287, doi:10.1021/jf901716j
- ^ Acid-catalyzed Epimerization of Kobophenol A to Carasinol B. Kejun Cheng, Gaolin Liang and Changqi Hu, Molecules 2008, 13(4), 938-942